| Case Name |
Fire and explosion in a butadiene rectifying column during preparation for turnaround shutdown maintenance |
| Pictograph |
|
| Date |
October 10, 1988 |
| Place |
Kawasaki, Kanagawa, Japan |
| Location |
Chemical factory |
| Overview |
As preparatory work for opening a distillation column of a butadiene plant during a turnaround shutdown, nitrogen purging, steaming, and air substitution were carried out in that order. During air substitution, three small explosions occurred in the column, and there was a fire inside the column. The butadiene concentration was high in the column, and polymers were generated during the operation period, which adhered to trays in the column. As an oxidation reaction of polymers with air started under substitution, a high-temperature arose. Remaining monomers or monomers generated from cracked polymers locally ignited at the high temperature. Then three small explosions occurred.
The countermeasures for the next turnaround shutdown is to pour water to prevent ignition of polymers, instead of carrying out steaming and air substitution after nitrogen purging, |
| Incident |
Explosion occurred in the No. 2 rectification column of the purification section of a plant which extracted and purified butadiene from the C4 fraction, which was obtained from an ethylene manufacturing plant as a by-product. To prepare a major inspection of the plant, hydrocarbon in the column was replaced with nitrogen after steaming. Then, displacement with air was executed for workers entering the column. At that time a small explosion occurred in the column. |
| Processing |
Manufacture |
| Individual Process |
Maintenance |
| Process Flow |
Fig2.Flow Sheet around the Rectifying Column
|
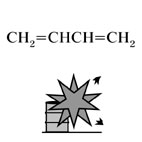
| Substance |
1,3-butadiene, Fig3 |
| Type of Accident |
Explosion, fire |
| Sequence |
On October 7th, 1988, the plant was shut down for the third turnaround shutdown. Nitrogen substitution for hydrocarbon gas and drawing-off of a liquid were carried out for regular repairs.
At 17:00, steam cleaning of the No. 2 rectification column started.
At 09:00 on October 8th, steam cleaning finished, and substitution with nitrogen gas and cooling started.
At 11:00 in October 9th, cooling with nitrogen gas finished, and manholes of the same column were opened.
At 18:00, air was introduced into the column and air substitution started.
At 01:15 and 01:45 on October 10th, two explosion sounds were detected. Although an operator checked the plant, no abnormality could be found.
At 02:05, the third explosion sound was detected. The shift leader in charge found smoke coming out from the upper manhole of the second rectification column during the field inspection after the explosion was detected. The cooling sprinkler system of the column started, and air that had been blown into the column was immediately switched to nitrogen. |
| Cause |
Highly concentrated butadiene often generates polymers and accumulates. Polymers also accumulated in the column where the accident occurred. Cooling with nitrogen after steaming was not executed sufficiently at the turnaround shutdown. A butadiene polymer was oxidized with air to generate heat, and cracked gas was generated by the heat. Therefore, a combustible gas-air mixture was formed in the column. It was estimated that the combustible gas-air mixture was ignited at the high temperature of the polymer, which accumulated heat from an exothermic oxidation reaction. |
| Response |
The operator stopped injecting air into the column and replaced it with nitrogen and started a cooling sprinkler system of the column. Fourteen public fire brigade engines, two private fire brigade engines, and three cooperating disaster prevention fire engines turned out. Four public fire engines, two self-defense fire engines, and one cooperating fire engine sprayed water from a fireplug and a water storage pond to fight the fire. |
| Countermeasures |
1. The purging method of an overhaul during a turnaround shutdown was returned to the old one. Air purging after substitution with nitrogen gas was stopped. Water is injected into the equipment to which the polymers might adhere to prevent ignition of the polymer.
2. Polymer-removing work should be done under sufficiently wet conditions, and water is gradually drawn off by adjusting the progress of removal work.
3. Generation of polymers is suppressed as much as possible. Oxygen that triggers polymerization is removed as much as possible at start-up. Therefore, cleaning with sodium nitrite and diethylhydroxylamine is done thoroughly, and addition of TBC (tertiary butyl catechol) as an antioxidant is strengthened. |
| Knowledge Comment |
1. If solid materials adhere to a vessel that has internal structures like a distillation column, it is very difficult to replace gas or liquid in the vessel completely by purge using other gas or liquid. The purging materials might avoid adhesive materials.
2. Therefore, for the system in which polymers might adhere, it is necessary to sufficiently consider the purging method before opening the system.
3. It is necessary to suppress formation of polymers as much as possible in a system on-stream where polymer formation is considered, Very small amounts of oxygen and moisture which remain in the equipment at the start may become an initiator, and polymers might be generated. In the process, a study of the use of oxygen scavengers, etc., will also be necessary.
4. Oxygen and water exist everywhere. It is necessary to take sufficient care of oxygen and water, because very hazardous conditions might be caused with very small amount of oxygen and water. |
| Background |
The most important factor of the accident would be underestimation of the hazard of butadiene polymers. The operation group etc. had to be conscious of the existence of butadiene polymers. Simultaneously, they had to know sufficiently that butadiene polymers are easily oxidized with air to generate heat. In addition, due to the existence of the polymer, flows of gas for purging and cooling were disturbed, and a channeling phenomenon in which gas for purging does not pass partially was easily caused. Therefore, it can be estimated that there was a part in which the temperature did not drop sufficiently before air substitution. Then, butadiene polymers in the rectification column caused an exothermic oxidation reaction. It is considered that a combustible gas-air mixture was generated from cracked gas generated at a high temperature, monomers (gas) confined within the polymer, or butadiene gas that remained in the channel during purging, etc., ignited and exploded.
In addition, where the accident occurred, the concentration of butadiene with a conjugated double bond was high, to induce easy formation of polymers.
The accident occurred at the third turnaround shutdown. To reduce a period for purging, and a workload of the plant, the purging method was changed. It seems to be a typical example of an accident caused due to an inadequate pre-study and insufficient knowledge.
Before the accident, another accident occurred at the similar plant in another company nearby. The danger of the process must be sufficiently examined. |
| Incidental Discussion |
There was a similar accident in a nearby factory on September 1st, 1979. Knowledge of this accident was not used. It is necessary to exchange accident information among companies.
At a place where butadiene concentration is high, polymers called a popcorn polymer and a diamond polymer are readily generated. The operating company and also the licensing company knew this well. So, there might have been some new polymer generation conditions. |
| Reason for Adding to DB |
The hazard of heat generation due to oxidation of a butadiene polymer |
| Scenario |
| Primary Scenario
|
Insufficient Analysis or Research, Insufficient Practice, Lack of Imagination, Poor Value Perception, Poor Safety Awareness, Inadequate Risk Recognition, Planning and Design, Poor Planning, Poor Planning for Purge, Non-Regular Action, Change, Change of Operation Procedure, Bad Event, Chemical Phenomenon, Abnormal Reaction, Secondary Damage, External Damage, Explosion
|
|
| Sources |
Kawasaki City, Fire fighting station. Prevention division. Peace section. N petrochemical Co., Ltd. K office. Outline of Butadiene manufacturing plant fire. Material of the Kawasaki City Complex safety countermeasure committee.
High Pressure Gas Safety Inst. of Japan. Butadiene manufacturing plant, Fire in the butadiene rectifying column during preparation of a turnaround shutdown. Accident examples of Petroleum refining and Petrochemical unit. pp. 123-124(1995).
|
| Physical Damage |
Deformation and/or falling from the top to the 38th stage of the tray (total of 85 trays) in the rectifying column. In particular, deformation was greater at the 30th stage. |
| Financial Cost |
About 2 million yen. (accident examples of petroleum refining and petrochemical unit) |
| Multimedia Files |
Fig3.Chemical formula
|
| Field |
Chemicals and Plants
|
| Author |
KOBAYASHI, Mitsuo (Office K)
TAMURA, Masamitsu (Center for Risk Management and Safety Sciences, Yokohama National University)
|
|